Abstract
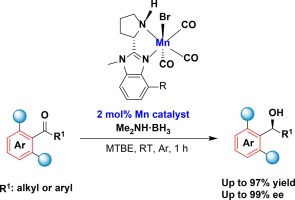
We describe here a manganese-catalyzed asymmetric transfer hydrogenation of hindered ketones containing 2,6disubstituted phenyl group. This method is enabled by using a readily available chiral aminobenzimidazole manganese(I) complex as the catalyst in combination with ammonia borane compounds as the hydrogen source (especially, dimethylamine borane), producing the chiral alcohols with up to 99 % ee and satisfied yields. Importantly, 2,6-disubstituted benzophenones could be smoothly transformed into the desired products with up to 95 % ee and satisfied yields.
Keywords Plus:ENANTIOSELECTIVE HYDROGENATION,LIGANDS,CRIZOTINIB,PHTHALIDES,ACCESS
Published in JOURNAL OF CATALYSIS,Volume 438;10.1016/j.jcat.2024.115680,OCT 2024


