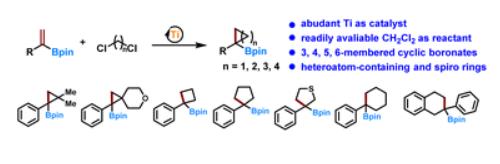
Abstract
Cyclic boronates are versatile synthons for organic synthesis and for introducing ring systems into bioactive molecules. Existing synthetic methods have narrow substrate scope and the synthesis of alpha-substituted cyclic boronates is still elusive. Furthermore, no general method for synthesizing cyclic boronates with different ring sizes and hetero atom containing rings is available. Herein, we present a new and general synthetic method for synthesizing alpha-substituted cyclic boronates. Our approach has the advantage of using earth-abundant Ti as the catalyst and readily available dihaloalkanes, such as dichloromethane, as the reactant. Cyclic boronates that are otherwise difficult to access, such as alpha-substituted cyclic boronates with three-, four-, five-, and six-membered rings, heteroatom-containing rings, and cyclic boronates with spiro rings, are readily obtained.
Keywords Plus:TITANOCENE(II)-PROMOTED REACTION,ENANTIOSELECTIVE HYDROBORATION,COPPER(I)-CATALYZED REACTION,CYCLOPROPANATION,INHIBITORS,OPTIMIZATION,BORYLATION,ALKENES,BOND
Published in CHEMICAL SCIENCE; 10.1039/d5sc01132a


