Abstract
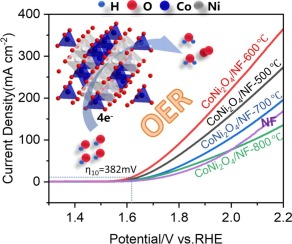
The oxygen evolution reaction (OER), a complex four-electron-proton-coupled process, plays a pivotal role in determining the efficiency of water electrolysis. The low cost and superior electrochemical kinetics of spinel catalysts endow them with promising potential for application in water electrolysis. Herein, spinel CoNi2O4 catalysts were synthesized on nickel foam (NF) via a facile sol-gel auto-combustion method for alkaline water electrolysis. The modulation of Ni3+/Ni2+ ratios via calcination temperature endowed CoNi2O4/NF-600 with excellent OER electrocatalytic performance. The CoNi2O4/NF-600 catalyst exhibited a low overpotential of 382 mV at 10 mA cm-2, an impressive Tafel slope of 86.3 mV dec-1, and electrocatalytic stability. Consequently, this work provides novel insights and approaches for the design and preparation of spinel catalysts, as well as regulation of the OER performance.
Keywords Plus:LDH
Published in MATERIALS LETTERS,Volume399;10.1016/j.matlet.2025.139089,NOV 15 2025


