Abstract
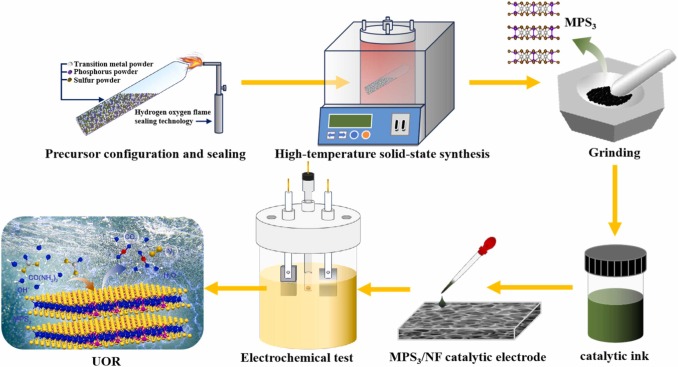
The replacement of the oxygen evolution reaction (OER) with the urea oxidation reaction (UOR) coupled with the hydrogen evolution reaction (HER) in water electrolysis systems is regarded as a highly promising strategy for reducing hydrogen production energy consumption, enhancing hydrogen efficiency, and even effectively treating urea wastewater. However, the design and fabrication of UOR catalysts in the UOR coupled HER catalytic water electrolysis system remains one of the significant challenges. Herein, we employed a facile, operable, and practical solid-state reaction method to synthesize two representative types of transition metal phosphosulfide (MPS3) catalysts, and the intrinsic and structural characteristics of these catalysts were systematically characterized. Subsequently, the MPS3 catalyst was deposited onto a nickel foam substrate (NF) to obtain the MPS/NF catalytic electrode. The UOR and OER performance of the MPS3/NF catalytic electrode was evaluated to elucidate the competitive mechanisms of UOR and OER within the MPS3 catalyst. Results indicated that the UOR driving potentials required for NiPS3/NF and FePS3/NF electrodes were individually reduced by 101 mV and 33 mV compared to the corresponding OER driving potentials under the condition of 100 mA/cm2, and NiPS3/NF and FePS3/NF electrodes could even achieve large UOR current density of 300 mA/cm2 at potentials as low as 1.630 V (vs. RHE) and 1.647 V (vs. RHE), respectively. In-situ Raman spectroscopy combined with XPS analysis revealed distinct catalytic pathways for NiPS3 and FePS3. During catalysis, NiPS3 undergoes surface reconstruction to form gamma-NiOOH, which exhibited superior selectivity toward urea oxidation, whereas FePS3 predominantly generated beta-FeOOH, whose activity was compromised by competing oxygen evolution, thereby conferring weaker UOR selectivity compared with NiPS3. For the UOR-coupled HER whole-cell urea electrolysis assessment, the NiPS3/NF and FePS3/NF whole-cell attained urea degradation efficiencies of 89.04 % and 69.61 %, respectively. This work provided a novel strategy and practical approach for the development of novel, efficient, and cost-effective MPS3 catalysts for UOR coupled with HER in the treatment of urea wastewater for synergistic hydrogen production.
Keywords Plus:OXYGEN EVOLUTION,HYDROGEN,EFFICIENT,ELECTRODES,NANOSHEET
Published in JOURNAL OF ALLOYS AND COMPOUNDS,Volume1040;10.1016/j.jallcom.2025.183646,SEP 23 2025


