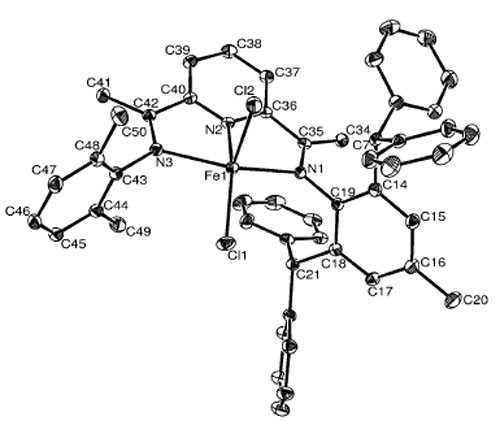
ORTEP drawing of complex Fe1 with thermal ellipsoids at 30% probability level. Hydrogen atoms have been omitted for clarity.
Researchers of the Institute of Chemistry and Lanzhou Institute of Chemical Physics of the CAS have performed ethylene polymerization using a series of 2-[1-(2,6-dibenzhydrylphenylimino)ethyl]-6-[1-(arylimino)ethyl]-pyridyliron(II) chlorides with the activity in the range of 107g PE mol-1 (Fe) h-1, which is the highest observed in iron procatalysts at elevated reaction temperatures such as 80℃ in the presence of MMAO and 60℃ in the presence of MAO, without any trace of ethylene oligomerization.
Beyond the high activity and industrially suitable temperature, the molecular weights and distributions of resultant polyethylenes could be controllable. The iron procatalysts are worthy of further investigation, and are potentially applicable for industrial consideration.
The emergence of bis(imino)pyridyliron(II) chlorides marked a milestone in iron-based procatalysts for ethylene polymerization and oligomerization. Previously, the researchers have studied iron(II) procatalysts employing new ligands. These catalytic systems are useful for either oligomerization or polymerization of ethylene. Impeding the progress of bis(imino)pyridyliron(II) procatalysts in ethylene polymerization, the critical problems are their deactivation and production of oligomers at elevated reaction temperatures. Though impressive bis(imino)pyridyliron procatalysts employing bulky anilines enhanced the thermostability, exploring more bulky anilines is still an attractive subject. In addition, the design of new bis(imino)pyridyliron procatalysts which will perform solely ethylene polymerization with high activity at elevated reaction temperature (suitable for industrial consideration between 60 to 80℃) is highly beneficial.
The work has been financially supported by the National Natural Science Foundation and the National High-tech R&D Program of China. The findings have been published in Chem. Commun. (Chem. Commun., 2011, 47, 3257–3259).


