Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics have developed a biaryl-bridged salalen titanium complex that exhibited high enantioselectivity in the epoxidation of a range of olefins with aqueous hydrogen peroxide as the oxidant.
The intramolecular dinuclear Ti catalyst possessing a biaryl bridge is highly efficient for the reaction of terminal aromatic olefins. Further studies on asymmetric catalysis and the mechanism of metal complexes bearing the biaryl-bridged salalen or salan ligands are in progress.
Optically active epoxides are very important molecules in a broad range of chemical transformations; these compounds are mainly produced by a critical process involving catalyzed enantioselective epoxidation reactions of olefins. Over the past three decades, many efforts have been devoted to the development of efficient catalysts for the enantioselective epoxidation of olefins.
The work has been financially supported by the Chinese Academy of Sciences and National Natural Science Foundation of China.
The work has been published in Eur. J. Org. Chem. (Eur. J. Org. Chem. 2011, 4289–4292).
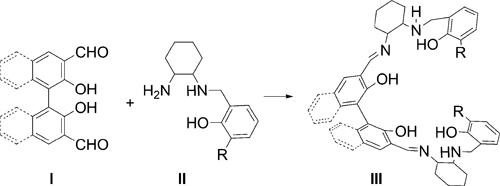
The synthetic route for biaryl-bridge salalen ligands.


