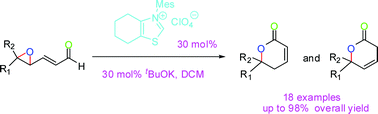
Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS have developed a highly efficient N-heterocyclic carbene catalyzed cascade epoxide-opening and lactonization reaction, which is a more direct and efficient way to construct the skeleton of dihydropyrones. A variety of readily accessible substrates underwent the reaction in good to excellent yield.
A mechanism for this reaction forming dihydropyrones, which proceeds via the NHC-catalyzed tandem epoxide-opening and lactonization reaction has also been provided. Further investigations into the precise mechanism of this reaction as well as the use of chiral carbenes as organocatalysts in other asymmetric reaction are currently underway in the laboratory.
N-Heterocyclic carbenes (NHCs) as organocatalysts have attracted a great deal of attention in the past decades, as they provide a broad range of useful synthetic transformations. However, the NHC-catalyzed redox reaction of γ-reducible-α,β-enals have received extremely rare consideration.
The work has received support from the Ministry of Science and Technology and National Natural Science Foundation of China.
The findings have been published in Org. Biomol. Chem. (Org. Biomol. Chem., 2011, 9, 5948–5950).