A facile and practical methodology for the synthesis of synthetically useful diarylmethanol-based 1,4-diols and enantiomerically pure BINOL-derived diols with axial and sp3-central chirality has been developed through neighboring lithium-promoted-[1,2]-Wittig rearrangement by researchers from Hangzhou Normal University and Lanzhou Institute of Chemical Physics of the CAS.
The chirality transfer process shows a broad substrate scope in terms of the aromatic ether substituent, which allows access to a broad of range of chiral 1,1′-binaphthalene-2--arylmethanol-2′-ols with excellent enantioselectivities (>99?% enantiomeric excess) and yields (84–96?%).
This should be considered as an available and attractive chiral source to design and prepare privileged ligands or catalysts.
The enantioselective-[1,2]-Wittig rearrangement was shown to be of particular value for the construction of chiral alcohols and their analogues from ethers or acetal compounds, with retention of configuration at the migrating carbon atom. Thus, it would be highly desirable to develop an enantioselective-Wittig rearrangement that could be used in the preparation of synthetically useful chiral products.
The facile preparation of unsymmetrical diols is an important and challenging goal in synthetic chemistry, because they are versatile building blocks for complex natural molecules. In particular, 1,4-diols (including diarylmethanolbased 1,4-diols) are widely used for the preparation of important heterocycles. Despite the proposal of various modified methods for the synthesis of 1,4-diols in the last decade, some of these still suffer from drawbacks in the key step, such as low yields, employment of expensive chemicals, tedious procedures, or multiple steps. Hence, development of new methodology to simultaneously address these issues was desirable.
The work has received support from the National Natural Science Foundation of China and Zhejiang Provincial Natural Science Foundation of China. The findings have been published in Chem. Eur. J. (Chem. Eur. J. 2011, 17, 2698 – 2703).
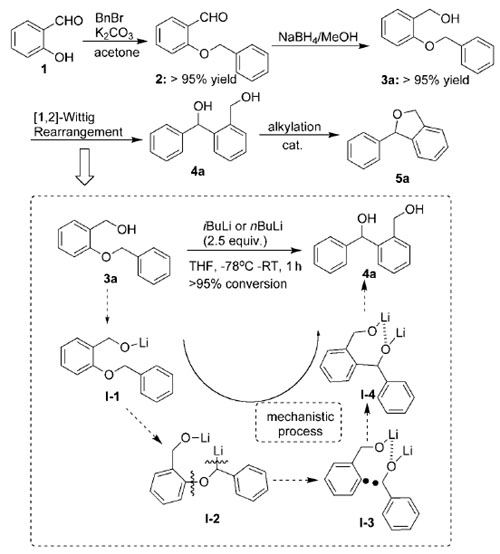
[1,2]-Wittig rearrangement of 2-benzyloxy-phenylmethanol


