In the field of supramolecular chemistry and crystal engineering, the design and assembly of metal–organic coordination polymers has attracted great attention due to their potential applications in many areas such as absorption, nonlinear optics, separation, and catalysis, etc. It is commonly known that many factors, including the chemical structure of the ligand, the coordination geometry preferred by the metal, the counterions, the metal-to-ligand ratio, and the experimental conditions, can affect the structure of the resulted complex, among which the nature of the ligand plays a crucial role in the construction and structural and functional tuning of coordination polymers. Therefore, systematic research of the above factors is an important issue toward controllable synthesis of desired frameworks.
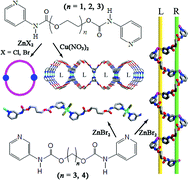
Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation of the Lanzhou Institute of Chemical Physics have four flexible bis(pyridylcarbamate) ligands L2 to L5 [L2=3-pyridyl-carbamic-acid-oxydi-1,2-ethanediylester, L3=3-pyridinyl-carbamic-acid-1,2-ethanediyl-bis(oxy-2,1-ethanediyl)ester, L4=3-pyridinyl-carbamic-acid-1,3-propanediylester and L5= 3-pyridinyl-carbamic-acid-1,4-butanediylester] with polyglycol spacers and the reactions of these ligands with zinc and copper salts afforded six metal–organic coordination architectures [Zn2Cl4(L2)2]·3H2O (1), [Zn2Br4(L2)2]·0.5H2O (2), {[Cu(L2)2(H2O)2](NO3)2·4H2O·4CH3OH}n (3), [Zn2Br4(L3)2] (4), (ZnBr2L4)n (5) and (ZnBr2L5)n (6)].
These compounds are structurally diverse, including four main structural motifs: three hydrogen-bonded 3D porous networks with different inner space volumes formed by 0D metallomacrocycles (compounds 1, 2 and 4), one 3D porous racemic framework 3 formed by 2D homochiral layers, one 1D meso-helical chain in compound 5 and one 1D linear chain of compound 6. The flexible ligands give rise to open 3D porous frameworks containing wide channels as well as helicity of the resulted polymers. Moreover, the 1D coordination polymers 5 and 6 further extend into 3D networks via classical and non-classical hydrogen bonding along with p–p stacking interactions. Hence extensive weak noncovalent interactions seem to play an important role in the crystal packing and stabilizing of the higher-dimensional supramolecular structures. The results also illustrate that the high flexibility of the ligands enables the formation of unusual structural topologies.
The work has been financially supported by the National Natural Science Foundation of China.
The findings have been published in CrystEngComm (CrystEngComm, 2011, 13, 5763–5772).