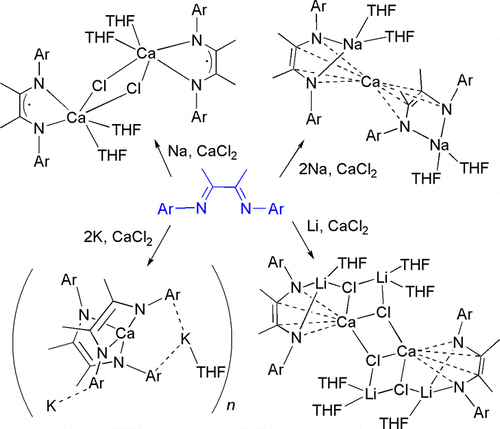
A series of calcium compounds with α-diimine ligands, [L-Ca-(μ2-Cl)(THF)2]2 (1, L = LiPr; 2, L = LMes), [(LiPr)2-2CaK2(THF)]n (3),[(LMes)2-Na(THF)2]2Ca (4), and [Ca(LiPr)2-Li(THF)(μ3-Cl)Li(THF)2-(μ3-Cl)]2 (5), where LiPr =[(2,6-iPr2C6H3)NC(Me)]2 and LMes= [(2,4,6-Me3C6H2)NC(Me)]2, have been synthesized by the reaction of the lithium, sodium, or potassium salts of the reduced ligands (L- or L2-) with calcium chloride by researchers from Northwest University, Lanzhou Institute of Chemical Physics and Fujian Institute on the Structure of Matters of the CAS.
Single-crystal X-ray diffraction analyses reveal that compounds 1 and 2 are isostructural dinuclear compounds bridged by chloride ions, compound 3 forms a left-handed 21 helical structure, while 4 features a metallocene-like structure, and 5 forms a laddered aggregate. The ligand exists as the doubly reduced dianionic form in 3-5 and appears as the radical anion in compounds 1 and 2. The coordination modes of the alkali metal and calcium ions in these complexes are discussed.
The organic compounds of calcium are of interest because of the close relevance of this element to its lighter congener magnesium, which forms a variety of important organometallic compounds, in particular the Grignard reagents. However, organocalcium compounds have been far less explored than the magnesium analogues due to the low reactivity of the calcium metal and high reactivity of the organocalcium products. On the other hand, this nature would facilitate certain applications such as promoting reactions in low temperature.
The N,N-chelating α-diimine agents are widely investigated for transition metal coordination compounds because of their use in catalysis. The steric and electronic properties of such ligands can be readily modified by attaching variable substituents on the carbon and nitrogen atoms of the NCCN backbone. In addition, as redox-active ligands, they can take one or two electrons to form the mono- and dianionic species upon reduction, which makes them particularly useful in the synthesis of subvalent metal complexes.
The findings have been published in Organometallics (Organometallics 2011, 30, 1599–1606).