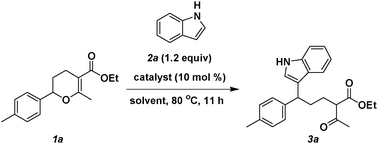
Ring-opening reactions of 2-aryl 3, 4-dihydropyrans with nucleophiles were reported by researchers from Huazhong University of Science and Technology and Lanzhou Institute of Chemical Physics of the CAS.
Indoles, 2-naphthol and 4-hydroxy-6-methyl-2-pyrone could be used to react with an appropriate dihydropyran to form the corresponding ring-opening products. Skeletons of the obtained products contain not only a core structure of the nucleophile, but also a moiety of b-dicarbonyl compound. Particularly, because of the presence of 1,3-diketone moiety, the ring-opening product could be further cyclised through a radical pathway initiated by Mn(OAc)3. Finally, a complex and valuable 2, 3, 4, 9-tetrahydro-1H-carbazole, was prepared in an overall yield of 61% through a three step synthesis starting from very simple substrates.
Dihydropyran and their derivatives are widely present in nature-occurring products. Some of dihydropyrans showed important biological and pharmaceutical activities, and therefore, their synthesis has attracted much attention. The researchers have previously reported a three-component reaction of olefins, formaldehyde and b-dicarbonyl compounds, which generated a variety of 2,5,6- trisubstituted 3,4-dihydropyran derivatives in high yields. In view of the fact that this reaction opens, indeed, an easy access to the dihydropyrans, use of these products in organic synthesis deserves further attention.
The work has received support from the National Natural Science Foundation of China, the Program for new Century Excellent Talents in the University of China and Specialized Research Fund for the Doctoral Program of Higher Education. The findings have been published in Chem. Commun. (Chem. Commun., 2011, 47, 4529–4531).


