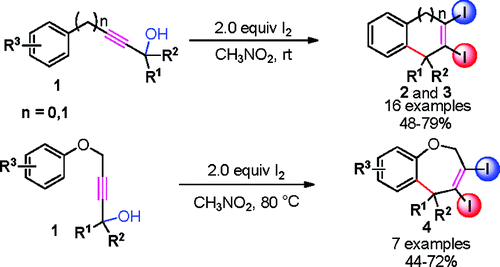
An efficient synthesis of substituted dihalogenated 1H-indenes, dihydronaphthalenes, and dihydrobenzo[ b]oxepines in moderate yields from simple aryl propargylic alcohols under mild reaction conditions has been developed by researchers from Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS.
The dihalogenated moiety can be readily introduced into the carbocycle and heterocycle in a position not easily obtained previously. The resulting diiodinated products can be used to prepare more complex products by using known organopalladium chemistry. In this reaction, both halogen atoms, such as I and Br, are used effectively and trace amount of water are necessary.
Recently, the intramolecular electrophilic cyclization of nucleophiles with alkynes has proven to be an effective method for the synthesis of carbocyclic and heterocyclic compounds. Many important heterocycles and carbocycles have been accessed using this protocol. However, the electrophilic carbocyclization with allenes has often been considered to be less attractive due to the lack of efficient control of the regio- and stereoselectivity.
The work has received support from the National Science Foundation National Basic Research Program of China. The detailed report has been published in Org. Lett. (Org. Lett., Vol. 13, No. 4, 2011).