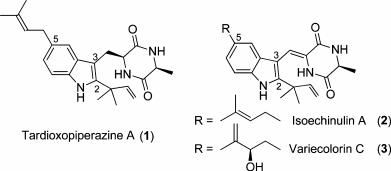
Researchers from Lanzhou University and Lanzhou Institute of Chemical Physics have reported the concise total syntheses of TardioxopiperazineA(1), IsoechinulinA(2), and VariecolorinC(3). Their synthetic route features three highlights: (1) application of a key intermediate for three molecules; (2) application of allylindium reagents; (3) construction of the diketopiperazine core with the reagent 1-(benzyloxy)-2-isocyanobenzene. The application of this route paves the way for the total syntheses of other isoechinulin-type alkaloids which are underway in their laboratory.
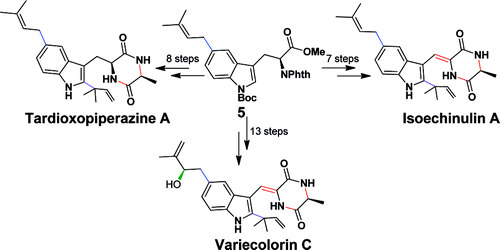
The structurally fascinating and biologically active metabolites TardioxopiperazineA(1), IsoechinulinA(2), and Variecolorin C (3), isolated from the Aspergillus species by Zhu and co-workers in 2007, are three new members of isoechinulin-type alkaloids (Figure 1). These 2,3,5-trisubstituted indole alkaloids contain three structural units: an indole, a 2-methyl-3-buten-2-yl, and a diketopiperazine. A bioactivity test shows that compounds 1-3 display radical scavenging activity, ultraviolet-A protecting activity, immunosuppressive activity, and antibacterial activity.
The work has received support from the National Natural Science Foundation of China. The detailed report has been published in Org. Lett. (Org. Lett., Vol. 13, No. 9, 2011).


