Marine sponges of the polyphyletic order “Lithistida” have been the source of a wealth of natural products with various biological activities. Neopeltolide (1) was isolated from a deep-water sponge of the family Neopeltidae by Wright and co-workers in 2007. Their bioactivity studies showed that that neopeltolide (1) exhibits significantly potent in vitro cytotoxicity toward several different cancer cell lines. Neopeltolide (1) also inhibited the growth of the fungal pathogen Candida albicans with a minimum inhibitory concentration of 0.62 μg/mL.
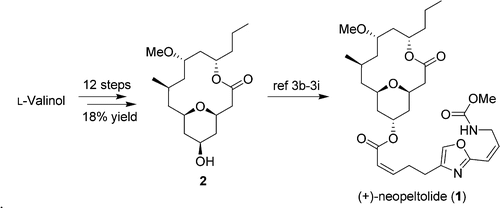
Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics of the Chinese Academy of Sciences (CAS) have achieved a concise formal synthesis of (+)-neopeltolide (1) in 12 steps (longest linear sequence) and 18% overall yield from commercially available L-valinol.
Notable features include high atom economy with minimized protective group manipulation, iridium-catalyzed double asymmetric carbonyl allylation, palladium-catalyzed intramolecular alkoxycarbonylation, ruthenium-catalyzed olefin isomerization, and ring-closing metathesis. This efficient pathway would enable large scale preparation of (+)-neopeltolide (1) offering convenience for further studies.
The study is expected to serve for further biological study and medicinal purposes.
The work has received support from the Ministry of Science and Technology, National Natural Science Foundation of China and the Program 111.
The findings have been published in Org. Lett. (Org. Lett., Vol. 13, No. 21, 2010.