An efficient approach to five-membered heterocyclic compounds allenes 2, diphenylvinyl-2,3-dihydro-1H-pyrrole (2y), polycyclic skeletons 3 and 4, and 1,3-dienyl-2,5-dihydro-1H-pyrrole (6as) by utilizing a Br?nsted acid catalyzed tandem cyclization reaction of hydroxylated enynes has been developed by researchers from Lanzhou University and Lanzhou Institute of Chemical Physics of the CAS.
This Br?nsted acid catalyzed domino process involves the formation of an allene carbocation intermediate, which can be readily trapped by olefins to give various novel five-membered heterocyclic skeletons. Furthermore, the simplest, and least expensive, Br?nsted acid shows excellent catalytic activity in the reaction with a low catalyst loading.
In recent years, the annulation of heterocycles, especially polyheterocyclic skeletons, has continued to attract the interest of synthetic chemists due to the number of these compounds that are extensively used as synthetic building blocks and also appear as subunits in many natural products that exhibit interesting biological activities. Today, the main challenge that organic chemists face is the production of these architecturally complex molecules in facile and efficient ways. The cycloisomerization of enynes is one such method. However, most of these procedures require high loadings of expensive metal catalysts and ligands. Moreover, the number of accessible heterocyclic skeletons is limited. Therefore, new and effective protocols, involving atom economic, environmentally benign, and mild reaction conditions, for the straightforward synthesis of heterocyclic rings are still of high demand in modern organic synthesis.
Five-membered oxygen- or nitrogenated heterocycles are important intermediates for the synthesis of pharmaceutically and biologically active molecules. Some efficient synthetic routes to these skeletons have been reported, of which carbon–hetero formation and carbon–carbon formation catalyzed by transition metals are more valuable. In contrast, examples of the cyclization or cycloisomerization reactions of hydroxylated enynes catalyzed by Br?nsted acids have rarely been reported.
The work has received support from National Science Foundation and the Fundamental Research Funds for the Central Universities. The findings have been published in Chem. Eur. J. (Chem. Eur. J. 2011, 17, 305 – 311).
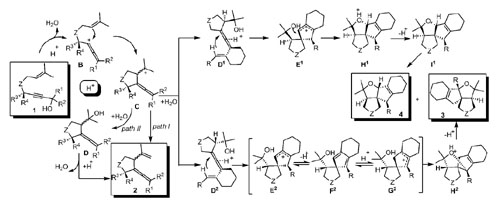
Proposed mechanism.


