The first efficient and convenient alternative for the preparation of a variety of biaryl ketones by using ligand-free Pd2(dba)3 as a catalyst under atmospheric pressure of carbon monoxide has been developed by researchers from Lanzhou University and Lanzhou Institute of Chemical Physics.
Compared with previous reports, several features of the present results were established: (1) The catalytic system is efficient and general not only for a broad range of aryl iodides and aryl boronic acids but also for various combinations of heteroaryl iodides with heteroaryl boronic acids. (2) A variety of functional groups, such as electron-donating groups, including CH3 and CH3O, electron-withdrawing groups, including CH3OCO, Br, and Cl, and even the strongly electron-withdrawing substituent NO2, are tolerated. (3) The reactions demonstrated high selectivity (up to 100% for the carbonylative Suzuki reaction of 4-iodoanisole with phenylboronic acid) for the catalytic system, and the important pharmaceutical intermediate 4-vinylphenyl 2-thienyl ketone could be prepared selectively in good yield. (4) This ligand free Pd2(dba)3-catalyzed carbonylative Suzuki coupling reaction provided high TONs up to 1700. (5) The catalyst can also be recycled.
Diaryl ketones are important building blocks in the synthesis of natural products and biologically active small molecules, and a number of approaches for their preparation have been introduced. This method allows the preparation of a variety of diaryl ketones/aryl heteroaryl ketones in good to excellent yields at low catalyst loadings under mild reaction conditions.
The work has received support from the Natural Science Foundation of Gansu Province. The findings have been published in Eur. J. Org. Chem. (Eur. J. Org. Chem. 2011, 2662–2667).
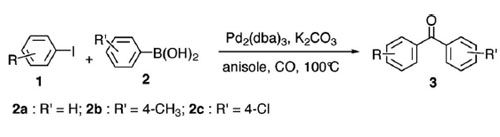
Suzuki carbonylative reaction of aryl iodides with arylboronic acids.(Reaction conditions: 1 (0.2 mmol), 2 (0.2 mmol), Pd2(dba)3 (1 mol-%), K2CO3 (3 equiv.), CO (balloon pressure), anisole (2 mL) at 100 °C for 20 h.)


