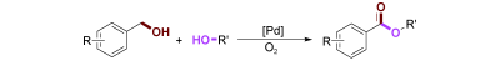
The first palladium-catalzyed direct aerobic oxidative esterification of benzylic alcohols with methanol and various long-chain aliphatic alcohols has been successfully developed by researchers from Lanzhou Institute of Chemical Physics and Wuhan University. By considering the effects of substitution and potential mechanistic pathways, researchers have been able to show the applicability of this method to a range of different substrates to give their corresponding esters in moderate to high yields. The challenging esterification reactions of long chain aliphatic alcohols were accomplished by using a P-olefin ligand to control the selectivity. The direct nature of this route and the use of O2 as the oxidant represent a step toward an environmentally benign and sustainable process.
Ester groups are among the highly important and abundant functional groups in chemistry and can be found in bulk chemicals, fine chemicals, natural products, and polymers. The existing methods for the preparation of esters require several reaction steps and produce unwanted by-products. Therefore, it is part of the challenges of green and sustainable chemistry to update these old processes.
Alcohols (as some of the most fundamental compounds) are usually readily available as bulk chemicals. Traditionally, alcohols could be converted into esters by multiple steps. However, direct conversion of alcohols into esters in the presence of catalysts could represent a step forward toward green, economic, and sustainable processes.
The findings have been published in Angew. Chem. Int. Ed. (Angew. Chem. Int. Ed. 2011, 50, 5144 –5148).


