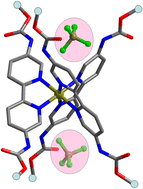
A series of 5,5′-dicarbamate-2,2′-bipyridine ligands and their mono-, bis-, and tris-chelating transition metal complexes were synthesized by researchers from Northwest University, Lanzhou University, Lanzhou Institute of Chemical Physics and University of Duesseldorf.
The carbamate functionality is not involved in metal coordination but participates in hydrogen bonding with various anions or solvents. In particular, the tris(chelate) complexes display the expected guest inclusion behavior within the clefts formed by three ligand arms. Moreover, the anion-binding ability of the photoactive tris(chelate) RuII complexes in solution was investigated by fluorescence emission spectroscopy, and they are found to exhibit prominent selectivity for SO42- ions in their fluorescence response.
The work has received support from National Natural Science Foundation of China. The findings have been published in Dalton Trans. (Dalton Trans., 2011, 40, 5687–5696).


