Oxidation reactions play an important role in organic chemistry research and the organic chemical industry, and many oxidation methodologies and reagents have been developed to date. Although numerous stoichiometric inorganic oxidants have been traditionally employed in oxidation reactions, there are considerable drawbacks such as their high cost, toxicity, and serious environmental effluents. Therefore, many efforts have been focusing on the activation and the application of molecular oxygen under mild condition.
Researchers at the Zhejiang University of Technology and Lanzhou Institute of Chemical Physics have a new catalytic oxidation system using catalytic amounts of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) and tert-butyl nitrite with molecular oxygen serving as the environmentally benign, terminal oxidant.
The catalytic oxidation system was established from the aerobic oxidation of non-sterically hindered benzylic alcohols and applied in the oxidative deprotection of PMB ethers. Under the optimal reaction conditions, a variety of PMB ethers can be deprotected in high conversions to their corresponding alcohols with high selectivities.
This aerobic oxidative deprotection can tolerate a broad range of other protecting groups under the reaction conditions.
The work has received support from the National Natural Science Foundation of China and the Zhejiang Provincial Natural Science Foundation of China.
The findings have been published in Adv. Synth. Catal. (Adv. Synth. Catal. 2011, 353, 3031 – 3038).
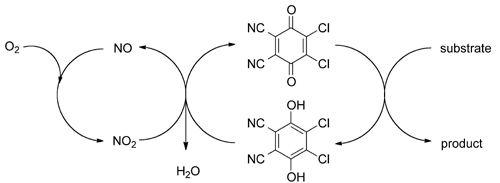
Proposed DDQ-mediated aerobic oxidation reactions.


