Magnesium compounds play indispensable roles in organic and organometallic chemistry, as they can be employed as convenient precursors for the synthesis of other products. The structures and reactivity of MgII complexes with N-donor ligands, have been reported previously, such as the homoleptic L2Mg and LMg(solvent)n complexes. However, monomeric magnesium complexes with N-aryl-substituted α-diimine ligands are rather rare considering the wide application of such ligands in the coordination with a variety of metal ions, from transition and rare earth to main group metals. Moreover, these ligands are redox noninnocent and can accept one or two electrons to form the monoanion and dianion, which in turn can serve as reductants in the synthesis of some low-valent complexes.
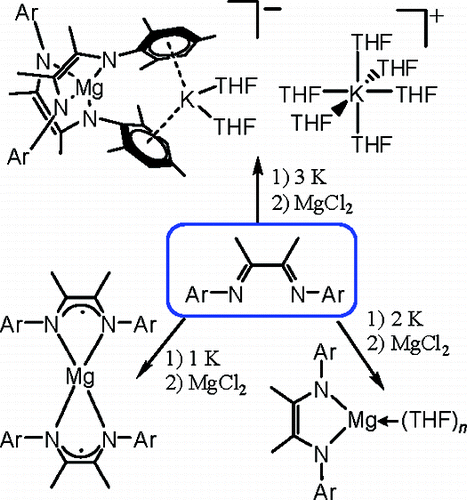
Researchers from the Lanzhou Institute of Chemical Physics and Nanjing University have synthesized a series of magnesium complexes ([(LiPr)2?Mg-(THF)2]·THF (2), [(LMes)2?Mg(THF)3] (3), [(LiPr)?2Mg]·THF (4), [(LMes)?2Mg] (5), and [(LMes)2?2Mg(η6:η 6-K(THF)2)][K(THF)6]·(THF)2 (6) (LiPr= [(2,6-iPr2C6H3)NC-(Me)]2), LMes= [(2,4,6-Me3C6H2)NC(Me)]2)) with reduced α-diimine ligands by the reaction of the neutral ligand, MgCl2, and potassium metal.
By varying the equivalents of K (to L), the α-diimine ligands accepted one or two electrons and formed the dianion (in 2, 3, and 6) or the monoanion (in 4 and 5) in the resulting complexes. However, the initial divalent magnesium ion retained its oxidation state of +2, which is different from the case of the MgI?MgI-bonded compound. Variable structures were obtained upon changing the amount of the reducing agent K and ligand substituents, and the effects of these factors have been discussed.
The work has received support from the National Natural Science Foundation of China. The findings have been published in Organometallics (Organometallics 2011, 30, 6071?6070.