Direct C–H bond functionalization towards C–C and C–heteroatom (N, O and S etc.) bond formation promoted by transition metals has attracted extensive attention in the past decades. However, most efforts were focused on noble metal catalysts, such as Ru, Rh, Pd, etc. However, few examples have been reported regarding Fe-catalysed direct oxidative C–H functionalizations.
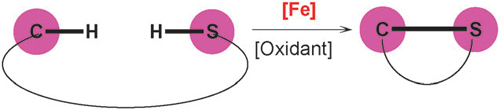 |
| Intramolecular C–S bond formation via C–H and S–H activation. |
Benzothiazoles are a very important class of heterocycles in the pharmaceutical area. Generally, oxidative cyclization is an efficient approach for the synthesis of thiobenzanilides by using various oxidants. However, the pre-functionalization of the starting materials in this protocol limited its application. It is no doubt that the direct oxidative intramolecular C–S bonds formation via C–H functionalization would be an attractive approach to synthesize benzothiazole.
Researchers from Wuhan University and Lanzhou Institute of Chemical Physics have developed an efficient iron-catalysed C–H functionalization/C–S bond formation under mild conditions. This transformation could be conveniently carried out affording various benzothiazoles in moderate to excellent yields.
Preliminary mechanistic studies revealed that the reaction required the co-existence of substrate, oxidant, FeCl3 and pyridine. Kinetic studies indicated that pyridine was crucial for the high selectivity of this transformation and the reaction was first order in the substrate and zero-order in oxidant Na2S2O8.


