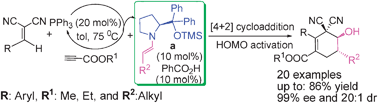
Researchers from the Lanzhou University and the Lanzhou Institute of Chemical Physics have disclosed the synthesis of dicyano-2-methylenebut-3-enoates as novel Diels–Alder dienes via a new PPh3-catalyzed addition reaction, and presented an unprecedented PPh3-catalyzed addition/all-carbon-based asymmetric IEDDAR sequence reaction for the first time, affording the products in high levels of enantio- and diastereoselectivity.
The catalytic asymmetric Diels–Alder reaction (DAR) has been recognized as one of the most powerful and convergent strategies for the stereoselective construction of six-membered functionalized cyclic frameworks, often containing multiple stereocenters. Due to its importance and versatility in the synthesis of diverse natural products, organic chemists are paying increasing attention to the development of novel asymmetric methodologies in this field. Catalytic asymmetric variants of these [4+2] reactions have been achieved for different diene–dienophile combinations, showing in several instances outstanding synthetic utility. On the other hand, compared with other classic reactions, it presents more difficulties due to the governing strict principle of generation of DAR including the suitable matching of diene with dienophile in accordance with the electronic orbital theory.
The work has received support from the National Natural Science Foundation of China and the National S & T Major Project of China. The findings have been published in Chem. Commun. (Chem. Commun., 2011, 47, 8289–8291).