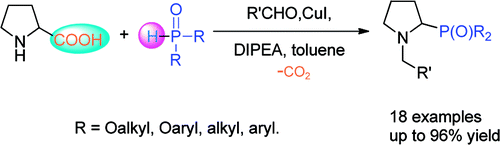
A copper/DIPEA-catalyzed, aldehyde-induced tandem decarboxylation-coupling of natural α-amino acids and phosphites or secondary phosphine oxides has been developed by researchers from the Lanzhou University and Lanzhou Institute of Chemical Physics.
In this process, a broad range of potential ligands for organic synthesis and biologically important non-natural amino acid derivatives (tertiary amino phosphorus compounds) can be readily accessible. The use of natural α-amino acids as materials, cheap copper salt as the catalyst, and the site-specific functionalization are some of the numerous advantages of this new method.
Phosphorus-containing compounds have received intense interest in recent years because of their wide applications. In particular, α-amino phosphonates have been widely used owing to their extensive role as antibacterial agents, antiviral agents, and enzyme inhibitors and their catalytic antibody activities. Although many methods have been developed, the researchers aim to develop a new way of the synthesis of α-amino phosphonates.
The work has received support from the National Natural Science Foundation of China and National S&T Major Project of China. The findings have been published in J. Org. Chem. (J. Org. Chem. 2011, 76, 6426–6431).