The biaryl structural motif is a predominant feature in many pharmaceutically relevant and biologically active compounds. The biaryl compounds are accessible through a large variety of metal-catalyzed cross-coupling reactions. At present, an attractive and good alternative is the direct arylation of aromatic C-H bonds with aryl halides in the presence of catalytic transition metals.
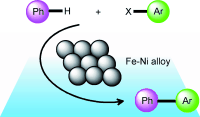 |
Researchers at the State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have developed a high yielding, mild, magnetic, Fe-Ni alloy-catalyzed direct arylation of unactivated benzene with aryl iodides without the requirement of a directing group. The precursors are cheap, and the method is simple. Notably, the catalyst exhibited excellent activity for the direct arylation of unactivated arenes and could be reused eight times without significant loss in catalytic activity. This approach is promising in terms of sustainability and practical applications in comparison to previous methods.
The work has received support from the Chinese Academy of Sciences and the National Natural Science Foundation of China. The findings have been published in ChemCatChem (ChemCatChem 2012, 4, 192 – 195).


