Catalytic asymmetric construction of tetrasubstituted carbon stereocenters is a challenging objective in chemical synthesis. The asymmetric conjugate addition of nucleophiles to acceptors such as β,β-disubstituted α,β-unsaturated carbonyl compounds is one of the most reliable approaches toward this challenge. To date, most studies have been focused on quaternary all-carbon stereocenters. By contrast, reports on building tetrasubstituted carbon stereocenters with nucleophiles of other elements including phosphorus nucleophiles have been much less developed yet.
Researchers from Lanzhou University and Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have reported the first conjugate addition of dialkyl phosphine oxides to β,β-disubstituted α,β-unsaturated enones and N-acylpyrroles.
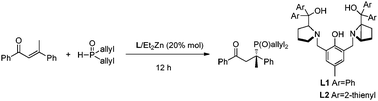 |
Dialkyl phosphine oxides were found as a class of highly reactive nucleophiles in the catalytic asymmetric phospha-Michael reaction with β,β-disubstituted α,β-unsaturated carbonyl compounds. The products bearing tetrasubstituted carbon stereocenters were obtained in high yields with excellent enantioselectivities (up to 499% ee). A relatively wide range of substrates scope was achieved in this process.
The work has received support from the National Natural Science Foundation of China, the Key National S&T Program "Major New Drug Development’’ of the Ministry of Science and Technology. The findings have been published in Chem. Commun. (Chem. Commun., 2012, 48, 889–891).


