Indolines are structural scaffolds frequently found in important pharmaceuticals and biologically active natural compounds. Inspired by Nature’s efficiency of selective indole oxidation and as part of their efforts in developing efficient methodologies for the functionalization of indoles, researchers from Wuhan University and Lanzhou Institute of Chemical Physics aim to discover a convenient and versatile method towards the synthesis of 2,3-disubstituted indolines directly from the oxidation of widely available indole derivatives.
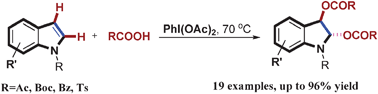 |
Recently, they have developed an efficient diacyloxylation of indoles with PhI(OAc)2 as the oxidant in the presence of carboxylic acid as the solvent. Interestingly, only trans diacyloxylated indolines were obtained, revealing a high stereo-selectivity. Moreover, a broad range of functional groups were well tolerated under these reaction conditions. On the basis of our observations, it is clear that both the electronic properties of the N-protecting groups of indoles and the acidity of the reaction media play important roles in the selectivity of this transformation. Related studies into asymmetric diacyloxylation of indoles and other heterocycles are underway in their laboratory.
The work has received support from the Changjiang Scholars and Innovative Research Team in University, National Natural Science Foundation of China and Wuhan University. The findings have been published in Chem. Commun.(Chem. Commun., 2012, 48, 3239–3241).


