Bio-oil is a renewable resource derived from biomass, which is environmental benign and has been regarded as a potential source for bio-hydrogen production. However, bio-oil is a complex mixture of organic compounds and its steam reforming is characterized with lots of difficulties. Acetic acid is one of the main acids in bio-oil and a safe hydrogen carrier due to its non-inflammable nature. The Ni-based catalysts are more promising in industrial application, due to their much lower cost. However, Ni-based catalyst is not selective and subjected to coking, which lowers the efficiency of steam reforming.
Researchers at State Key Laboratory for Oxo Synthesis, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have carried out steam reforming of acetic acid over a series of Ni/ZrO2 catalysts to measure the effects of nickel loading on distribution of the reforming products and coke formation.
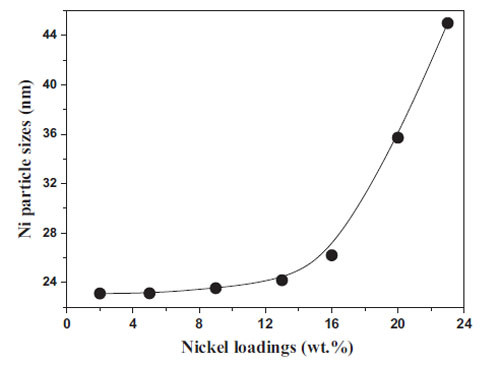 |
| Effect of Ni loadings on Ni particle sizes. |
It has been found that Ni (≤13 wt.%)/ZrO2 catalysts do not contain enough active metal sites for steam reforming of both acetic acid and organic by-products. Ni (≥20 wt.%)/ZrO2 catalysts can effectively catalyze steam reforming but lack selectivity, since methanation and reverse water gas shift reactions are promoted, leading to low hydrogen yields. Ni (16 wt.%)/ZrO2 catalyst is the most selective one, due to its low activity to the secondary reactions that contribute to by-product production. Coke formation is suppressed with the increase of nickel loading up to 16 wt.%, and then restarts to increases with the further increase of nickel loading. Polymerization of acetone is the main route for coke deposition over the Ni (≤13 wt.%)/ZrO2 catalysts. Methane decomposition and CO disproportion are the two main routes for coke formation over the Ni (≥20 wt.%)/ZrO2 catalysts, and methane contributes more to coke formation than CO. In addition, activity of Ni/ZrO2 catalyst towards the secondary reactions such as methanation, reverse water gas shift reaction, methane decomposition, and CO disproportion are closely related to nickel loading and nickel particle sizes.
The work has received support from National Program on Key Basic Research Project of China (973 Program). The findings have been published in Applied Catalysis A: General(Applied Catalysis A: General417– 418 (2012) 281– 289).


