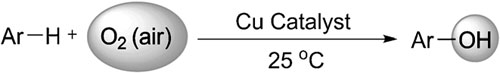
Copper-catalyzed “oxygenase-type” oxidation of arenes and heteroarenes.
Although transition-metal-catalyzed direct functionalization of C-H bonds has emerged as a powerful method to construct new C-C and C-heteroatom bonds, oxidative functionalization of C-H bonds under mild conditions (at room temperature and using air as the oxidant) still remains a very challenging field. In particular, the synthesis of phenols by direct oxidation of arenes with O2 as the oxidant is still regarded as one of the main challenges for catalysis, and is rarely reported. Thus, it is still necessary to develop a powerful synthetic method for the oxidative hydroxylation of CH bonds under mild reaction conditions.
Researchers at Lanzhou University and Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have reported a copper-catalyzed “oxygenase-type” oxidation of arenes and heteroarenes at room temperature under air (balloon). This reaction involves oxygen-atom transfer from O2 in the air onto the substrate. A reaction mechanism that includes a C-H activation process and sequentially facile oxidation of ArCuI species, has been corroborated through a series of deuteration and kinetics experiments. Moreover, CuOtBu is believed to be the active copper species and could be generated in situ from CuCl2 and NaOtBu by a SET process. Nevertheless, the following catalytic cycle follows a typical organometallic mechanism. Therefore, this copper-catalyzed aerobic oxidation reaction is believed to incorporates a novel combination of SET initiation and organometallic catalytic cycle. Further improvement of the catalytic efficiency of this transformation and expansion of the substrate scopes are ongoing.
The findings have been published in Angew. Chem. Int. Ed.(Angew. Chem. Int. Ed.2012, 51, 4666 –4670).


