 |
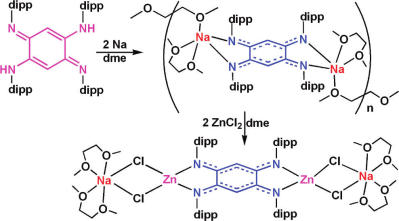
Researchers at State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have developed the reactions of the neutral azophenine ligand dippdabqdiH2 with Lin''Bu or Na metal which yielded the first alkali metal complexes (1 and 2) with the bridging [dipp-dabqdi]2?ligand. Reaction of the lithium and sodium complexes with anhydrous ZnCl2 afforded three ion-contacted zinc complexes. In these complexes, a complete electronic delocalization of the quinonoid π-system occurs between the metal centers over the two N=C-C=C-N halves of the [dipp-dabqdi]2? ligand. In addition, the unoxidized tetramine ligand 1,2,4,5-tetrakis- (2,6-diisopropylamino)benzene (dipp-tabH4) was also isolated, which reacted with K metal to give the potassium complex (3) of the doubly deprotonated ligand [dipp-tabH2]2?. The electronic structures of the complexes were confirmed by DFT calculations. These alkali metal complexes with the bridging [dipp-dabqdi]2? ligand may be suitable starting materials for the preparation of new dinuclear metal complexes possessing electronic communications mediated by the bridging ligand.
The work has received support from the National Natural Science Foundation of China and the findings have been published inInorg. Chem.(Inorg. Chem.2012, 51, 5889?5896).


