Esterification is a fundamental transformation in chemistry. The essential feature of esterification that particularly distinguishes it from other reactions lies in its broad utilization in industry. Up to now, esterification only largely occurs between carboxylic acid derivatives and alcohols. Aldehydes are also readily available and are bulk scale raw chemicals in industry. Theoretically, esterification of aldehydes with alcohols is an attractive alternative. However, current methods to esterify an aldehyde with an alcohol require oxidation to the acid in advance. As a result, multiple steps are involved with production of toxic irremovable byproducts, and is incongruent with the current demand of environmentally benign processes. Therefore, successful efforts during the past ten years have been focused on direct esterification of aldehydes with alcohols in the presence of an oxidant and catalyst. However, challenges still remain.
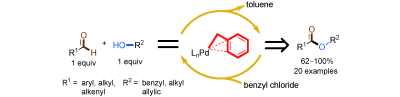 |
Researchers at Wuhan University and Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences (CAS), have demonstrated that a covalently bound benzyl ligand promotes the selective palladium catalyzed oxidative esterification of aldehydes with alcohols by using benzyl chloride as the oxidant. The covalently bound benzyl group is a carbon ligand which provides an h3- coordination effect on palladium. The study of the substrate scope showed that various aldehydes and alcohols were selectively converted into the corresponding esters in high selectivity and good to excellent yields by using benzyl chloride as the oxidant. Moreover, the ratio of the aldehyde and alcohol is 1:1. Neither of the substrates needs to be excess. Under solvent-free reaction conditions large-scale reactions were carried out in the presence of a low catalyst loading. Importantly, the effect of the benzyl ligand provides information for the further design of ligands in achieving selective palladium-catalyzed oxidative esterification.
The findings have been published in Angew. Chem. Int. Ed.(Angew. Chem. Int. Ed.2012, 51, 5662 –5666).


