With the development of the modern industry, large amounts of the greenhouse gases are released into the atmosphere, eventually leading to the global warming, namely, greenhouse effect. Carbon dioxide and methane are commonly recognized as two sorts of the most important greenhouse gases committed to the greenhouse effect. How to effectively realize the mitigation of these two greenhouse gases, especially carbon dioxide, becomes an imminent problem to be solved. The catalytic process of carbon dioxide reforming of methane (CRM) provides a potential route to simultaneously transform these two greenhouse gases into more valuable synthesis gas. Whereas, the rapid deactivation of the catalysts deriving from the carbon deposition and the thermal sintering of the metallic active centers hinders the large scale industrial application. Therefore, the development of catalysts high catalytic activity is of great significance.
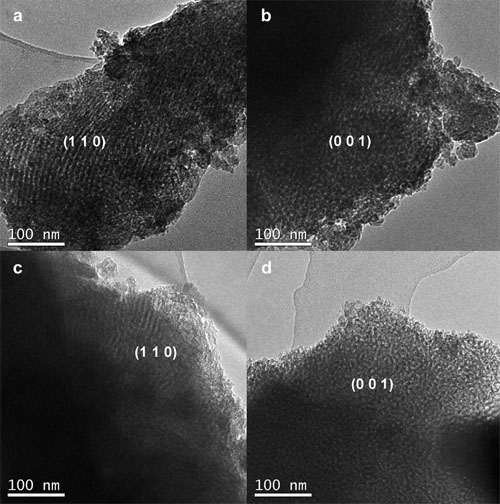 |
| TEM images of the X%Ni/OMA catalysts: (a) and (b) 7%Ni/OMA, (c) and (d) 10%Ni/OMA. |
Researchers at State Key Laboratory for Oxo Synthesis and Selective Oxidation, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, have synthesized ordered mesoporous alumina with large specific surface area, big pore volume, uniform pore size and excellent thermal stability via improved evaporation-induced self-assembly (EISA) strategy. The obtained mesoporous material was used as the carrier of the Ni based catalysts for carbon dioxide reforming of methane.
These mesoporous catalysts performed high catalytic activity and long stability. Typically, the catalytic conversions of the CH4 and CO2 were greatly close to the equilibrium conversion and no deactivation was observed during the 100h long lifetime test. The advantageous structural properties of ordered mesoporous alumina contributed to high dispersion of the Ni particles among the mesoporous framework, which further accounted for the good catalytic activity due to more “accessible” Ni active sites for the reactants. The “confinement effect” of the mesopores could effectively prevent the thermal sintering of the Ni nanoparticles to some extent, committed to its long-term catalytic stability. Besides, the mesoporous catalysts possessed enhanced ability to withstand coke, although not any modifiers had been added. Properties of the coke over the mesoporous catalyst were also carefully investigated. Therefore, the ordered mesoporous alumina was a promising catalyst support for the carbon dioxide reforming with methane.
The work has received support from the National Basic Research Program of PR China and the National Natural Science Foundation of China. The findings have been published in International Journal of Hydrogen Energy(International Journal of Hydrogen Energy37 (2012) 7497-7511).


