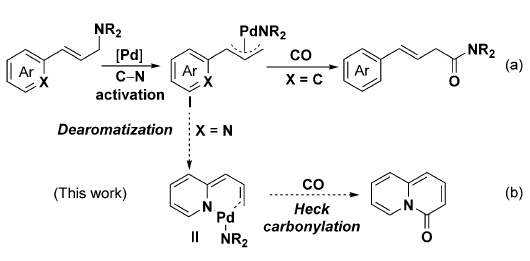
Abstract: A fundamentally novel approach to bioactive quinolizinones is based on the palladium-catalyzed intramolecular cyclocarbonylation of allylamines. [Pd(Xantphos)I-2], which features a very large bite angle, has been found to facilitate the rapid carbonylation of azaarene-substituted allylamines into bioactive quinolizinones in good to excellent yields. This transformation represents the first dearomative carbonylation and is proposed to proceed by palladium-catalyzed CN bond activation, dearomatization, CO insertion, and a Heck reaction.
Key words: CN activation; cyclocarbonylation; dearomatization; palladium; quinolizinones
Published in ANGEWANDTE CHEMIE-INTERNATIONAL EDITION, 54 (37):10912-10916; 10.1002/anie.201504805 SEP 7 2015


