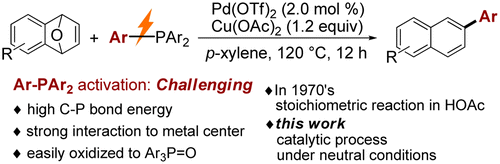
Abstact:A new approach was developed for Pd(II)-catalyzed aromatic coupling of oxabenzonorbornadienes with triarylphosphines as both ligands and aryl donors. Diverse functional groups including halo- (F-, Cl-, and Br-), CF3-, and furyl groups are well tolerated. For unsymmetrical triarylphosphines, the migration ability of aryls is consistent with the electronic property of substituents and maintains the order EDG-Ar > H-Ar > EWD-Ar (EDG means electron-donating group, EWG means electron-withdrawing group). A preliminary mechanistic study was also disclosed.
Key words Plus:CARBON-PHOSPHORUS BOND; PALLADIUM-CATALYZED PHOSPHINATION; COORDINATED PHOSPHINES; ASYMMETRIC CATALYSIS; OXABICYCLIC ALKENES; CLEAVAGE; COMPLEXES; LIGANDS; RHODIUM; ORGANOCATALYSIS
Published ORGANIC LETTERS, 17 (18):4628-4631; 10.1021/acs.orglett.5b02366 SEP 18 2015


