Abstract: A series of palladium-NHC complexes was prepared and their catalytic activity in the oxidative aminocarbonylation of alkynes with amines has been developed using oxygen as the benign oxidant, leading to an efficient approach toward the formation of a wide range of 2-ynamides with high atom efficiency. Utilizing the distinctive reactivity of this palladium-NHC catalysis, both the aryl and the alkyl of the alkynes could be efficiently incorporated into the 2-ynamide. Mechanistic studies provide some evidence to support that amido-Pd could be transformed into amido-Pd-alkynyl.
KeyWords Plus: SONOGASHIRA COUPLING REACTIONS; AMIDE BOND FORMATION; MOLECULAR-OXYGEN; PHOSPHINE-FREE; ARYL IODIDES; CARBONYLATION REACTIONS; CATALYZED OXIDATION; TERMINAL ALKYNES; SUZUKI-MIYAURA; EFFICIENT
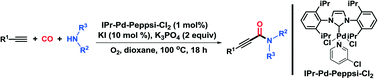
Published in CATALYSIS SCIENCE & TECHNOLOGY, 5 (10):4750-4754; 10.1039/c5cy00993f 2015


