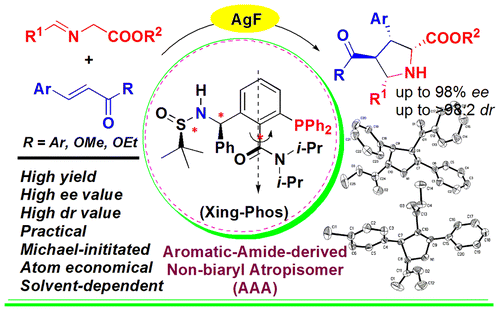
Abstract: In this work, we have successfully determined that the aromatic amide-derived nonbiaryl atropisomer/silver complex (silver-Xing-Phos) is an effective catalyst system for the solvent-dependent exo-selective cycloaddition of glycine aldimino esters with chalcones or less-reactive methyl cinnamates to give the corresponding chalcone- or cinnamate-derived pyrrolidines with multiple stereogenic centers in good yields and high diastereoselectivities as well as excellent enantioselectivities. Remarkably, it is the first example of highly enantioselective silver-catalyzed [3 + 2] cycloaddition of methyl cinnamates with glycine aldimino esters.
Key words:asymmetric catalysis, pyrrolidines, silver, homogeneous catalysis, atropisomers, cycloaddition
Published in ACS CATALYSIS, 5 (10):6016-6020; 10.1021/acscatal.5b01685 OCT 2015


