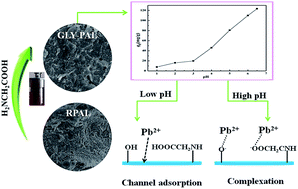
Abstract: As the materials of "green 21st century material worlds", natural silicates have received unprecedented attention by virtue of their abundance, low-cost, stability, and non-toxic and eco-friendly nature compared to other synthetic materials. With the aim to develop a new hybrid silicate adsorbent with improved adsorption properties, the naturally abundant palygorskite (PAL) was functionalized with glycine (GLY) via a simple one-step hydrothermal process and used for capturing Pb(II) ions from aqueous solution. The main reaction parameters, e.g., the pH values of the reaction medium, solid-to-liquid ratio, reaction time and dosage of GLY, were systematically optimized, and the as-prepared adsorbent was characterized using X-ray diffraction (XRD), field-emission scanning electron microscopy (FESEM), transmittance electronic microscopy (TEM) and Fourier transform infrared spectroscopy (FTIR) techniques. The results reveal that the PAL crystal was converted to a hybrid silicate material with the assistance of GLY, and simultaneously the functional groups were introduced during the hydrothermal reaction, which caused an evident enhancement in the adsorption capacity of PAL for Pb(II) ions from 55.76 mg g(-1) to 123.24 mg g(-1). Almost 99.60% of Pb(II) could be captured and removed from a 40 mg L-1 Pb(II) solution using the as-prepared GLY-PAL silicate adsorbent, which is obviously higher than the 83.85% achieved by raw PAL. The intensified complexation of the functional groups on the silicate with Pb(II), the electrostatic attraction and the pore adsorption are responsible for the enhancement in the adsorption capability.
KeyWords Plus:COORDINATED DIVALENT-CATIONS; ACID-ACTIVATED PALYGORSKITE; AQUEOUS-SOLUTIONS; METHYLENE-BLUE; KAOLINITE CLAY; HEAVY-METALS; AMINO-ACIDS; ADSORPTION; REMOVAL; MONTMORILLONITE
Published in RSC ADVANCES, 5 (117):96829-96839; 10.1039/c5ra17724c 2015


