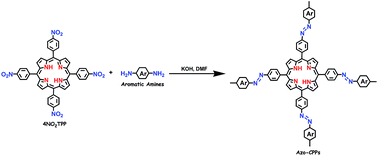
Abstract: A series of azo-bridged, aromatic, nitrogen-rich, microporous covalent porphyrinic polymers (Azo-CPPs) were synthesized through a metal catalyst-free direct coupling of a kind of tetranitro building block, 4NO(2)TPP, and several diamine or tetraamine compounds, under basic conditions. The Azo-CPPs were all amorphous and exhibited surface areas between 197 to 591 m(2) g(-1). The effect of building blocks on the pore properties was discussed. Meanwhile, the capacity of adsorption of CO2 for all the Azo-CPPs was studied and the CO2 uptake capacity was between 7.36% and 9.22% at 273 K and 1.10 bar. The CO2/N-2 selectivities were measured between 27.4-53.9 at 273 K and 31.2-107.8 at 303 K, respectively, showing an increase in selectivity with increasing temperature.
KeyWords Plus: POROUS ORGANIC POLYMERS; CARBON-DIOXIDE CAPTURE; CONJUGATED MICROPOROUS POLYMERS; BENZIMIDAZOLE-LINKED POLYMERS; TRIAZINE-BASED FRAMEWORKS; ENERGY-STORAGE; SURFACE-AREA; GAS-STORAGE; ADSORPTION PROPERTIES; NANOPOROUS POLYMER
Published in RSC ADVANCES, 5 (117):96871-96878; 10.1039/c5ra17671a 2015


