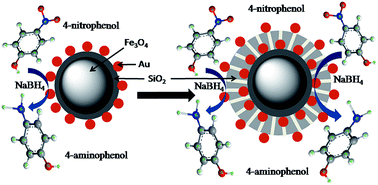
Abstract: In this paper magnetic nanocomposite catalysts, with a single gold catalytic layer (Fe3O4@SiO2-Au and Fe3O4@SiO2-Au@mSiO(2)), double gold catalytic layers (Fe3O4@SiO2-Au@mSiO(2)-Au), and with one gold layer and one silver layer (Fe3O4@SiO2-Au@mSiO(2)-Ag), were prepared through simple steps. Different techniques were used for characterization of the as prepared nanocomposite catalysts, such as transmission electron microscopy (TEM), X-ray diffraction (XRD), a vibrating sample magnetometer (VSM), energy-dispersive X-ray spectroscopy (EDX) etc. The catalysts were used for the catalytic reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in the presence of NaHB4. All the nanocomposite catalysts showed very good catalytic activity; however the nanocomposites with double catalytic layers (Fe3O4@SiO2-Au@mSiO(2)-Au and Fe3O4@SiO2-Au@mSiO(2)-Ag) showed excellent catalytic activity for the reduction reaction of 4-nitrophenol. Due to the magnetic core, the nanocomposite catalysts showed magnetic properties and can be easily separated and recycled many times without any loss in activity. In the case of nanocomposite catalysts with Au nanoparticles protected by silica (Fe3O4@SiO2-Au@mSiO(2)) indicated better recyclability compared to other nanocomposites.
Key wordsplus: IN-SITU SYNTHESIS; NEAR-IR ABSORPTION; GOLD NANOPARTICLES; P-NITROPHENOL; AG NANOPARTICLES; SUPPORTED AU; REDUCTION; SHELL; 4-NITROPHENOL; STABILITY
Published in RSC ADVANCES, 5 (121):99697-99705; 10.1039/c5ra18119d 2015


