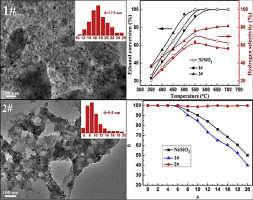
Abstract: Nickel is an active component of catalyst for hydrogen production from ethanol steam reforming (ESR). However, Ni based catalyst usually meets severe deactivation due to coking. Normally, Ni catalyst regeneration could be achieved by heating coked catalyst in the oxygen contained atmosphere gas to remove coked carbon by oxidation of carbon. During this regeneration process, dispersed Ni particles are easily segregated because burning of carbon generates huge amount of heat and leads to uncontrollable temperature increase. In this paper, a new regeneration method of Ni catalyst was developed by re deposition of another portion of Ni on deactivated Ni/SiO2 catalyst (denoted as catalyst 2) instead of O-2 combustion of the deactivated catalyst (denoted as catalyst 1). H-2-TPR results indicated that the metal-support interaction of catalyst 2 was stronger than that of catalyst 1, leading to higher metal dispersion and smaller Ni particles size over catalyst 2. The H-2 chemisorption and HRTEM results indicated the dispersion of catalyst 2 reached 19.3%, much higher than 9.4% of catalyst 1. The dimension of late-loaded Ni particle was around 9.5 nm, even smaller than that of catalyst 1 (17.5 nm), corresponding to the high catalytic activity and stability of catalyst 2. The ethanol conversion of catalyst 1 and catalyst 2 were 76.5% and 94.6% at 500 degrees C, respectively. After short term of 20 h test, the ethanol conversion of catalyst 2 still remained, while that of the catalyst 1 decreased to about 40% of initial activity under same reaction condition (T = 550 degrees C, S/C = 7.5, LHSV = 4.1 h(-1) and P = 1 atm.). (C) 2016 Hydrogen Energy Publications LLC.
Keywords: Regeneration of Ni catalyst; Ethanol steam reforming; Hydrogen production; Metal-support interaction; High dispersion
Published in INTERNATIONAL JOURNAL OF HYDROGEN ENERGY, 41 (32):13993-14002; 10.1016/j.ijhydene.2016.05.042 AUG 24 2016


