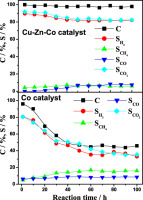
Abstract: In this study, steam reforming of acetic acid over trimetallic catalysts (Cu-Zn-Co), bimetallic catalysts (Cu-Zn, Cu-Co, Co-Zn), and monometallic catalysts (Cu, Zn, Co) for hydrogen generation have been investigated. The trimetallic Cu-Zn-Co catalysts were more active than the bimetallic or monometallic catalysts, showing higher capability to suppress the side reactions and higher resistivity towards the coke formation. The-superior activity of Cu-Zn-Co catalyst was due to the synergistic effects between the three metal species. Cu, Zn, and Co species, which played quite different roles in steam reforming of acetic acid. Cu was the important species to suppress the formation of CO, possibly via the water gas shift reaction. Co was the main active species for the reforming of the organics including acetic acid and the reaction intermediates such as acetone and methane, which determined the overall activity of the catalyst. Zn played the roles largely as the promoter to enhance the catalytic activity, especially at the low reaction temperature (<573 K). (C) 2016 Hydrogen Energy Publications LLC.
Keywords: Acetic acid; Hydrogen; Steam reforming; Cusingle bondZnsingle bondCo; Synergistic effects
Published in INTERNATIONAL JOURNAL OF HYDROGEN ENERGY, 41 (32):13960-13969; 10.1016/j.ijhydene.2016.05.066 AUG 24 2016


