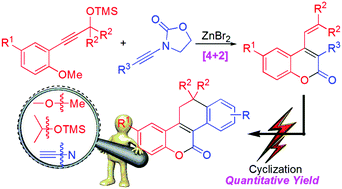
Abstract: Facile carbocation-initiated [4+2] cycloaddition of o-anisole-substituted propargyl silyl ethers and ynamides led to the formation of 4-vinylcoumarins. Subsequent intramolecular cyclization of 3-aryl-4-vinylcoumarins afforded polycyclic coumarin derivatives 11,12-dihydronaphtho[1,2-c] chromen-5-ones in excellent yields under mild photo-irradiated conditions established by fluorescence analysis-oriented screening.
KeyWords Plus:PROPARGYLIC ALCOHOLS; FLUORESCENT-PROBES; SILYL ETHERS; YNAMIDES; ACID; REARRANGEMENTS; ALDEHYDES; CATALYST
Published in CHEMICAL COMMUNICATIONS, 52 (74):11131-11134; 10.1039/c6cc05698a 2016


