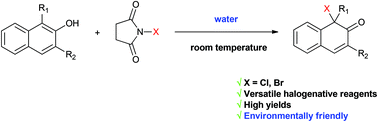
Abstract: A direct halogenative dearomatization of substituted 2-naphthols was achieved by NXS (X = Cl, Br) in environmentally friendly water at room temperature. The reaction exhibited broad substrate scope, convenient operation and high yields (up to 99%), thus providing a facile route to dearomatic products and complex polycyclic frameworks.
KeyWords Plus:HYDROXYLATIVE PHENOL DEAROMATIZATION; ONE-POT SYNTHESIS; BETA-KETO-ESTERS; ASYMMETRIC DEAROMATIZATION; N-BROMOSUCCINIMIDE; AQUEOUS-MEDIA; INTRAMOLECULAR DEAROMATIZATION; ENANTIOSELECTIVE SYNTHESIS; OXIDATIVE DEAROMATIZATION; NAPHTHOL DEAROMATIZATION
Published in GREEN CHEMISTRY, 18 (20):5485-5492; 10.1039/c6gc01673a 2016


