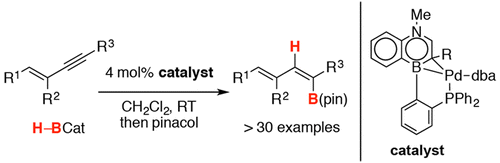
Abstract: A concise synthesis of monobenzofused 1,4-azaborine phosphine ligands (Senphos) is described. These Senphos ligands uniquely support Pd-catalyzed trans-selective hydroboration of terminal and internal 1,3-enynes to furnish corresponding dienylboronates in high efficiency and diastereoselectivity. X-ray structural analysis of the Senphos-Pd(0) complex reveals a kappa(2)-P-eta(2)-BC coordination mode, and this isolated complex has been shown to serve as a competent catalyst for the trans-hydroboration reaction. This work demonstrates that the expanded chemical space provided by the BN/CC isosterism approach translates into the functional space in the context of stereoselective catalytic transformations.
KeyWords Plus: CROSS-COUPLING REACTIONS; B-N; TERMINAL ALKYNES; C-C; INTERNAL ALKYNES; LIGAND STRUCTURE; BORON; ANALOGS; INDOLE; HETEROSUPERBENZENES
Published in JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, 138 (44):14566-14569; 10.1021/jacs.6b09759 NOV 9 2016


