Abstract: Thanks to the facile imine-enamine tautomerization, the beta,gamma-unsaturated hydrazones have been successfully utilized as surrogates of aminodienes for realizing the Pd-catalyzed tandem aminomethylamination/aromatization reaction with aminals via C-N bond activation. This direct and operationally simple protocol provides a fundamentally novel strategy to synthesize aromatic heterocycles from alkenes in the absence of external oxidant and base. Mechanistic studies suggested that aminal not only functioned as an aminomethyl source but also acted as formal oxidant and inner base to promote the aromatization.
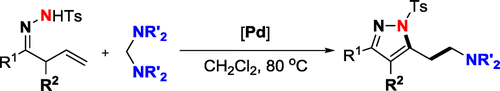
KeyWords Plus: BETA,GAMMA-UNSATURATED HYDRAZONES; HETEROCYCLES; CHEMISTRY; STRATEGY; ALKENES; CYCLOISOMERIZATIONS; FUNCTIONALIZATION; INSERTION; ALCOHOLS
Published in ORGANIC LETTERS, 18 (21):5736-5739; 10.1021/acs.orglett.6b03001 NOV 4 2016


