Abstract: The catalytic construction of benzimidazoles using CO, as a carbon source represents a facile and sustainable approach to obtaining these valuable compounds. Herein, we describe the B(C6F5)(3)-catalyzed synthesis of benzimidazoles via cyclization of o-phenylenediamines with CO2 and PhSiH3. This metal-free catalytic route achieves the desired products in high yield under convenient reaction conditions and is applicable to a broad substrate scope. A plausible mechanism for the reaction involving a frustrated Lewis pair pathway is proposed based on spectroscopic characterization (e.g., C-13 NMR) of the reaction intermediates.
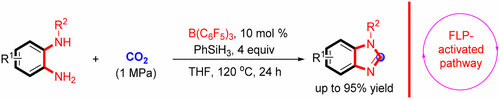
KeyWords Plus: FRUSTRATED LEWIS PAIRS; HIGHLY ENANTIOSELECTIVE HYDROGENATION; CARBON-DIOXIDE; FORMIC-ACID; CATALYZED SYNTHESIS; BUILDING-BLOCK; VISIBLE-LIGHT; BORANE; REDUCTION; COUMARINS
Published in ORGANIC LETTERS, 18 (24):6316-6319; 10.1021/acs.orglett.6b03030 DEC 16 2016


