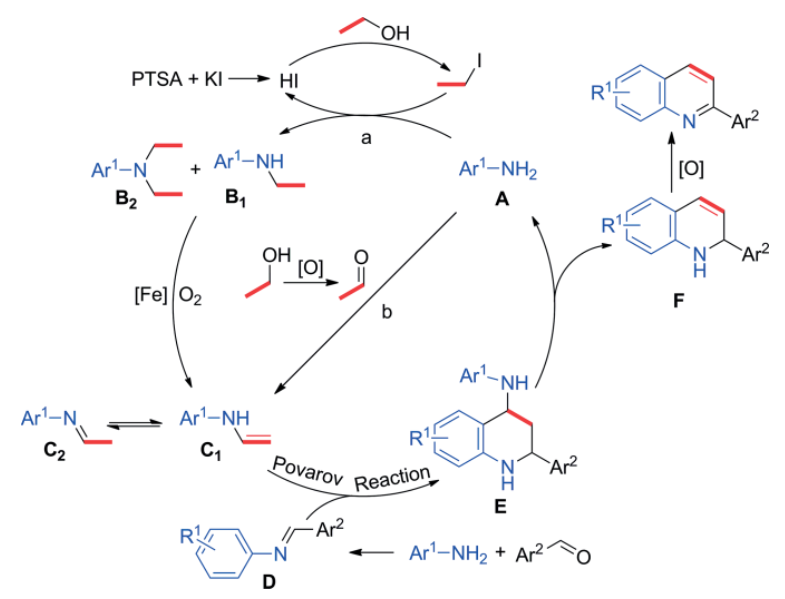
Abstract: An atom-economic and efficient approach to the synthesis of 2-arylquinolines has been developed. The protocol involves an iron-catalysed cascade N-alkylation/aerobic oxidation/ Povarov reaction, and the desired quinolines were prepared in moderate to excellent yields from readily accessible anilines, aldehydes, and EtOH/nPrOH, with water as the only side-product. The aniline substrates also act as a recyclable transfer medium for EtOH/nPrOH through an in-situ N-alkylation/ oxidation process. This makes EtOH/nPrOH an economical and environmentally friendly precursor of alkenes as well as the solvent.
KeyWords Plus: CATALYZED DIRECT AMINATION; DIELS-ALDER REACTION; ONE-POT SYNTHESIS; C-C BOND; SUBSTITUTED QUINOLINES; ALLYLIC ALCOHOLS; 2-SUBSTITUTED QUINOLINES; ACRIDONE ALKALOIDS; ROOM-TEMPERATURE; TANDEM REACTION
Published in EUROPEAN JOURNAL OF ORGANIC CHEMISTRY, 2017 (3):618-625; 10.1002/ejoc.201601343 JAN 2017


