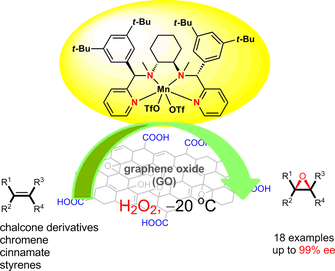
Abstract: Bioinspired manganese complexes of N-4 ligands and graphene oxide (GO) synergistically catalyze the highly enantioselective epoxidation of olefins (up to 99% ee), which is a rare example with GO as a co-catalyst in asymmetric catalysis. GO as a new and key additive not only successfully functions in catalytic amounts, but also has a positive effect for improving the enantioselectivity of the asymmetric epoxidation compared with the traditional stoichiometric organic carboxylic acid method [e.g., chalcone, 95% ee (3.5 mg GO) vs. 84% ee (5 equiv., 75 mg acetic acid), ethyl cinnamate, 84% ee (3.5 mg GO) vs. 19% ee (5 equiv., 75 mg acetic acid)]. The X-ray photoelectron spectroscopy (XPS) spectra of GO before and after the reaction indicate that the intensities of C-O become stronger after the reaction, which may have a certain relationship with hydrogen peroxide (H2O2) and gives a reasonable rationale for the large consumption of H2O2. Also, part of the hydrogen peroxide was used for the oxidation of GO.
KeyWords Plus: ELECTRON-DEFICIENT OLEFINS; NONHEME IRON CATALYSTS; HIGHLY ENANTIOSELECTIVE EPOXIDATION; PERACETIC-ACID EPOXIDATION; GRAPHITE OXIDE; STEREOSELECTIVE EPOXIDATION; ACETIC-ACID; N-4 LIGANDS; EFFICIENT CATALYSTS; ALKENE EPOXIDATION
Published in ADVANCED SYNTHESIS & CATALYSIS, 359 (3):476-484; 10.1002/adsc.201600848 FEB 2017


