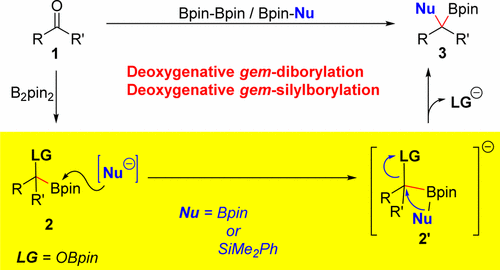
Abstract: A deoxygenative gem-diborylation and gem-silylborylation of aldehydes and ketones is described. The key for the success of this transformation is the base-promoted C-O bond borylation or silylation of the generated alpha-oxyboronates. Experimental and theoretical studies exhibit that the C-O bond functionalization proceeds via an intramolecular five-membered transition-state (9-ts) boryl migration followed by a 1,2-metalate rearrangement with OBpin as a leaving group. The transformation occurs with an inversion on the carbon center. Direct conversion of aldehydes and ketones to gem-diboron compounds was achieved by combining copper catalysis with this base-promoted C-OBpin borylation. Various aldehydes and ketones were deoxygenatively gem-diborylated. gem-Silylborylation of aldehydes and ketones were achieved which B2pin2 initially react with those carbonyls followed by a silylation with Bpin-SiMe2Ph.
KeyWords Plus:T ERT-BUTANESULFINYL ALDIMINES; COPPER-CATALYZED SYNTHESIS; AMINO BORONATE ESTERS; ASYMMETRIC-SYNTHESIS; HYDROXYBORONATE ESTERS; ORGANIC-SYNTHESIS; ROOM-TEMPERATURE; TERMINAL ALKYNES; FACILE SYNTHESIS; VINYL HALIDES
Published in JOURNAL OF THE AMERICAN CHEMICAL SOCIETY, 139 (14):5257-5264; 10.1021/jacs.7b02518 APR 12 2017


